● Intended Use
The kit intends for in vitro qualitatively detection of nucleic acid RNA of Respiratory syncytial virus (RSV) and Human parainfluenza virus (HPIV) 1/2/3 in oropharyngeal swabs. It can be used to assist in the diagnosis of respiratory infectious diseases.
The detection results are only for clinical reference, and aids in the diagnosis of respiratory infection if used in conjunction with other clinical and epidemiological information.
***For professional use
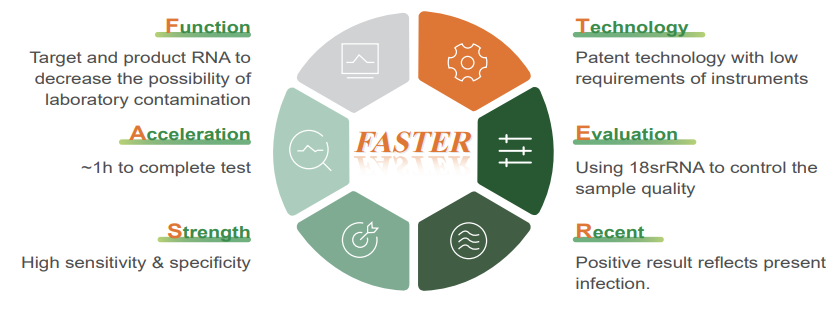
● Features
● Recommended Instrument

● Respiratory Tract Infections (RTIs)
Respiratory tract infections are infections of parts of the body involved in breathing, such as the sinuses, throat, airways or lungs. Due to the differences of infections organism, RTIs divided into upper and lower respiratory tract, symptoms include cold, bronchiolitis, pneumonia, croup, otitis media. Viruses are the most common cause of RTIs, such as influenza viruses, respiratory syncytial virus, chlamydia pneumoniae, human parainfluenza virus, and coronavirus.
Lower respiratory tract infections (LRTI) remained the world’s most deadly communicable disease. WHO (2019) reported that LRTI ranked as the 4th leading cause of death globally and 4th leading cause of disability-adjusted life years. Besides, Acute Respiratory Tract Infection (ARTI) is one of the most common infectious diseases and its incidence ranked as 1st among acute infectious diseases.
ZBI’s respiratory nucleic acid detection assay helps to faster the detection speed, to improve the sensitivity and specificity, to shorter the window period, and to decrease the cost.